Approximately 1.3 million heart valve replacements were performed worldwide annually, and the number of patients was expected to rise four to five times by 2050. Nowadays, mechanical valves are durable, but they are teratogenic during pregnancy and thus patients need lifelong therapy of anticoagulants, including vitamin K antagonists. Biological valves, though biocompatible, are prone to early calcification and leaflet degeneration, which occur during hospitalization, reoperations, and a short lifespan. The polystyrene hard domains in styrene-block-ethylene/butylene-block-styrene (SEBS) provide strength, durability, and flexibility to create physical cross-links in a constant elastomeric phase.
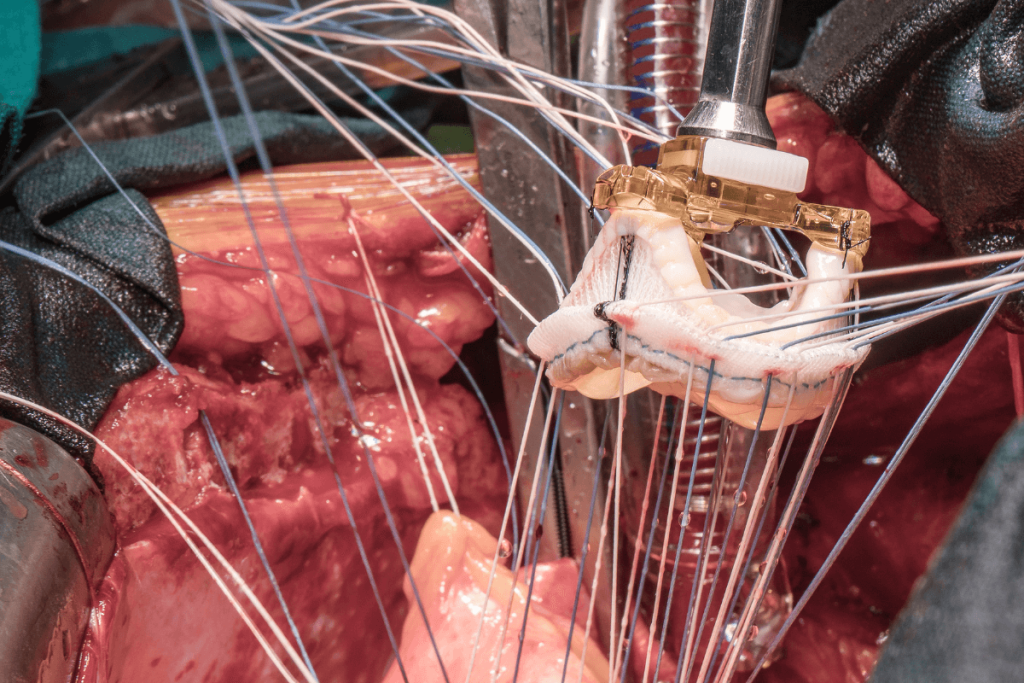
SEBS with 20%-33% styrene itself builds in a cylindrical microstructure that causes structural anisotropy with two orthogonal orientations. By using SEBS29 and SEBS20, scientists have created an aortic heart valve for research purposes. Using a juvenile sheep model, the current 6-month preclinical in vivo study evaluated the safety of SEBS materials indicated to create heart valves. The independent pathologists collected samples and scanned the heart, including the valve. Both the ventricular and atrial sides were captured in high resolution.
Histological analysis was conducted on central strips taken from each cusp. The amount of calcium and phosphorus in the residual portions of each cusp was measured. The presence or absence of calcification was determined by scoring high-resolution radiography pictures. An autosampler was used to filter and load a predetermined sample solution onto the column. The myocardium samples were incubated in 1% silver nitrate solution, while their calcification was evaluated by von Kossa staining.
The images were captured using an M8 Microscope to calculate the number of nuclei. The sample size (six animals) and the absence of a control group were determined after conversations with the regulator. In the mitral position, seven SEBS research-grade polymeric heart valves were implanted in seven animals. Out of these, four were normal and 3 were heparin-coated valves. Every test article was implanted in the designated position and location. There were no adverse events observed related to the test articles that were clinically significant during the implantation procedures.
From day 2 to day 181 following surgery, there was no deterioration of SEBS29 material in any of the seven prototypes. It also noted no variation from the non-implanted raw material. The electrolyte balance and glucose metabolism remained normal as biochemistry levels stayed within normal reference levels. All the animals maintained their typical appetites and weight gains and remained in good health.
Four animals made it to the last 180 ± 5 days timepoint; one required sacrifice on day 2 for unrelated reasons to SEBS. While one died on day 81 for unrelated causes, one required sacrifice on day 169 for reasons linked to a prototype malfunction.
The 6-month safety of SEBS29/SEBS20 material was confirmed by this preclinical in vivo investigation conducted on young sheep. The early investigation is important to check long-term durability, compatibility, and human function.
Reference: Ascione R, Stasiak JR, Baz-Lopez D, Serrani M, Moggridge GD. Material safety of styrene-block-ethylene/butylene-block-styrene copolymers used for cardiac valves: 6-month in vivo results from a juvenile sheep model. Eur J Cardiothorac Surg. 2025;67(8):ezaf266. doi:10.1093/ejcts/ezaf266












